Why Safety Matters in Monopolar RF Treatments
The global demand for non-invasive skin tightening treatments has grown rapidly over the past decade. Patients increasingly seek visible anti-aging results without surgery, needles, or downtime, making radiofrequency (RF)–based technologies one of the most established and trusted solutions in aesthetic practice. Among them, RF monopolare is recognized for its ability to deliver deep, uniform dermal heating and long-term collagen remodeling.
As the popularity of RF treatments continues to rise, safety has become a central concern for clinics, regulators, and patients alike. Effective outcomes must be supported by:
- Proven technology
- Clear regulatory positioning
- Proper patient selection and screening
- Correct clinical use by trained operators
Without these elements, even well-known technologies can carry unnecessary risk.
For professional clinics, safety is not only a medical or ethical issue—it is also a business and reputational priority. Adverse events, improper indications, or regulatory non-compliance can lead to patient dissatisfaction, legal exposure, and long-term damage to clinic credibility. This is why modern aesthetic practices increasingly prioritize devices that combine clinical effectiveness with predictable safety profiles and documented compliance.
Volnewmer RF is positioned specifically as a professional, clinic-grade monopolar RF system, intended for use by trained practitioners in medical aesthetic clinics and high-level beauty centers. It is not a consumer or home-use device. Its design, energy delivery principles, and regulatory classification reflect the expectations of professional environments where patient screening, informed consent, and standardized protocols are part of routine practice.
In the following sections, we will examine how Volnewmer RF addresses safety through technology design, CE compliance, contraindication management, and responsible clinical use, helping clinics deliver effective skin tightening treatments with confidence.
What Makes Volnewmer RF a Safe Technology?
Safety in aesthetic energy-based devices is not achieved by marketing claims alone. It is the result of well-understood physical principles, predictable tissue interaction, E controlled energy delivery. Volnewmer RF is built on these foundations, which is why monopolar radiofrequency has remained a core technology in professional aesthetics for decades.
2.1 Proven Monopolar RF Principles
Long history of radiofrequency use in aesthetics
Radiofrequency technology has been used in aesthetic and dermatologic applications for many years, with a well-documented safety profile when operated correctly. Unlike newer or experimental modalities, monopolar RF is supported by extensive clinical experience and long-term observation in professional settings.
This history provides practitioners with confidence in:
- Expected tissue response
- Typical side effects
- Appropriate treatment parameters
- Clear contraindication guidelines
Thermal energy based on tissue resistance, not light absorption
Volnewmer RF generates heat through electrical resistance within the tissue, rather than through light or chromophore absorption. This distinction is critical for safety.
Because RF energy does not rely on melanin, hemoglobin, or water as a target:
- There is no selective absorption by skin pigment
- The risk of pigment-related complications is reduced
- Treatment outcomes are more consistent across different skin tones
Adatto a tutti i tipi di pelle Fitzpatrick
Since monopolar RF is melanin-independent, Volnewmer can be safely used on all Fitzpatrick skin types (I–VI) when proper protocols are followed. This makes it particularly valuable for clinics serving diverse patient populations, where laser-based treatments may carry higher pigment-related risks.
2.2 Controlled Energy Delivery
Stable 6.78 MHz monopolar RF output
Volnewmer operates at 6,78 MHz, a frequency widely recognized for effective and controlled dermal heating. Stability at this frequency is essential for safety, as inconsistent energy output can lead to uneven heating or patient discomfort.
A stable RF signal allows practitioners to:
- Deliver predictable thermal effects
- Maintain consistent treatment quality
- Reduce the likelihood of localized overheating
Uniform dermal heating
Rather than concentrating energy at a single focal point, monopolar RF distributes heat uniformly throughout the dermal layer. This uniformity helps:
- Minimize hot spots
- Improve patient comfort
- Support even collagen remodeling across the treatment area
Designed to avoid epidermal overheating when used correctly
Volnewmer is engineered to prioritize controlled thermal transfer, helping protect the epidermis while allowing sufficient heat accumulation in the deeper dermis. When used according to recommended protocols—proper gel application, gradual energy adjustment, and continuous patient feedback—the risk of surface overheating is significantly reduced.
This design philosophy supports a balance between:
- Treatment effectiveness
- Patient comfort
- Clinical safety
CE Compliance and Regulatory Classification
Come il manufacturer of Volnewmer RF, Mico Aes places regulatory compliance and product safety at the core of our design, manufacturing, and delivery processes. From component selection to final testing, Volnewmer RF is developed to meet the expectations of professional aesthetic clinics operating in regulated markets.
3.1 CE Declaration of Conformity (EU)
CE-marked as a non-medical aesthetic device
Volnewmer RF is manufactured by Mico Aes and supplied with a CE Declaration of Conformity as a non-medical aesthetic device. This confirms that the device complies with applicable European Union directives for safety, performance, and electromagnetic compatibility within its intended cosmetic scope of use.
Manufactured under ISO-compliant quality systems
At Mico Aes, Volnewmer RF is produced under ISO-compliant quality management systems, ensuring:
- Controlled and standardized manufacturing processes
- Component traceability
- Documented quality inspection and testing procedures
These systems are implemented to support consistent product quality and long-term operational reliability in professional clinic environments.
Meets essential EU safety and EMC requirements
Volnewmer RF has been designed and tested to meet essential EU requirements related to:
- Electrical safety
- Electromagnetic compatibility (EMC)
- Safe operation alongside other clinic equipment
This helps ensure stable performance and reduces the risk of electromagnetic interference in professional settings.
3.2 Non-Medical Aesthetic Positioning
No medical claims
As the manufacturer, Mico Aes clearly positions Volnewmer RF as a non-medical aesthetic device. The product does not make medical claims and is not promoted for diagnosis, treatment, or prevention of any disease.
No treatment of disease
Volnewmer RF is not intended for medical therapy. It is designed exclusively for cosmetic and aesthetic applications focused on skin appearance.
Intended for cosmetic skin tightening and rejuvenation
The intended use of Volnewmer RF is limited to:
- Cosmetic skin tightening
- Improvement of skin texture and firmness
- Non-invasive aesthetic rejuvenation
This clear intended-use definition supports regulatory transparency and responsible clinical communication.
3.3 International Market Considerations
Common acceptance in EU aesthetic clinics
CE-marked, non-medical monopolar RF devices manufactured under ISO quality systems are widely accepted in EU aesthetic clinics and professional beauty centers. Volnewmer RF is developed to align with these market expectations.
Local regulations may vary
While CE compliance supports access to many international markets, local regulatory requirements may differ by country or region. Clinics and distributors are responsible for confirming local compliance obligations, including licensing, registration, and operational scope.
Manufacturer support: documentation and training
As the factory, Mico Aes provides comprehensive support to distributors and clinics, including:
- CE Declaration of Conformity
- Technical documentation and user manuals
- Safety guidelines and contraindication information
- Training materials for proper operation
This manufacturer-level support helps ensure Volnewmer RF is used safely, compliantly, and consistently across global markets.
Non-Invasive and Needle-Free Treatment
Volnewmer RF is designed by Mico Aes as a fully non-invasive aesthetic technology, eliminating many of the risks commonly associated with invasive or minimally invasive procedures. Its safety profile is rooted in the fact that treatment is delivered externally and contact-based, without breaching the skin barrier.
No injections
Volnewmer RF does not involve injections of any kind. There is no penetration of the skin and no delivery of substances beneath the surface.
No needles
The treatment is completely needle-free. This removes risks associated with bleeding, bruising, needle-related anxiety, and needle-stick injuries for operators.
No drug or serum delivery
Volnewmer RF does not deliver medications, drugs, vitamins, or active substances into the skin. RF energy acts purely through controlled thermal stimulation of tissue.
No skin penetration
Because the epidermal barrier remains intact throughout treatment, the risk of:
- Infezione
- Delayed healing
- Post-treatment wound care
is significantly reduced compared to invasive procedures.
Reduced infection and cross-contamination risk
By avoiding skin penetration, Volnewmer RF minimizes cross-contamination risks and simplifies hygiene protocols in clinical environments. Standard surface disinfection and proper operator practices are sufficient when the device is used according to manufacturer guidelines.
5. Contraindications for Volnewmer RF Treatment
Although monopolar RF technology has a well-established safety profile, proper patient screening remains essential. As the manufacturer, Mico Aes emphasizes that Volnewmer RF should only be used after careful evaluation of contraindications and precautions.
5.1 Absolute Contraindications
Volnewmer RF treatment should not be performed in the following cases:
- Pacemakers or implanted electronic devices
RF energy may interfere with electronic implants, posing a serious safety risk. - Gravidanza
As a precautionary measure, RF treatments are contraindicated during pregnancy. - Protesi metalliche nell'area di trattamento
Metal can alter RF energy distribution and increase the risk of localized overheating. - Active cancer in the treatment area
RF treatment should not be applied over areas with known or suspected malignancy. - Severe cardiac conditions
Patients with serious heart conditions should not undergo RF treatments without explicit medical clearance.
5.2 Relative / Precautionary Contraindications
In the following situations, treatment may require postponement, modification, or additional medical evaluation:
- Epilepsy
Careful assessment is required due to potential sensitivity to electrical stimulation. - Severe diabetes with impaired sensation
Reduced thermal sensitivity may increase the risk of unrecognized overheating. - Active skin infections or inflammation
Conditions such as dermatitis, bacterial infections, or severe acne should be resolved before treatment. - Open wounds or unhealed scars
RF should not be applied to areas where the skin barrier is compromised. - Recent surgical procedures in the treatment area
Adequate healing time must be allowed before RF exposure.
Final assessment is always at the practitioner’s discretion, based on professional judgment, patient history, and local regulatory requirements.
6. Patient Selection and Pre-Treatment Screening
- Importance of medical history review
- Informed consent documentation
- Managing patient expectations
- When to postpone or refuse treatment
7. Clinical Use Guidelines for Safe Operation
7.1 Operator Training
Safe and effective monopolar RF treatment begins before the device is activated. Proper patient selection and structured pre-treatment screening are essential to minimize risk, ensure appropriate indications, and achieve predictable outcomes. As the manufacturer, Mico Aes recommends that Volnewmer RF treatments only be performed following a standardized screening process.
Importance of medical history review
A thorough review of the patient’s medical history helps identify contraindications and precautionary conditions. This review should include:
- Implanted electronic devices or metal implants
- Pregnancy status
- Cardiovascular history
- Neurological conditions
- Recent surgical procedures
- Current skin conditions in the treatment area
Documented medical history review supports both patient safety and professional accountability.
Informed consent documentation
Prior to treatment, patients should receive clear information regarding:
- The nature of monopolar RF treatment
- Expected sensations during treatment
- Potential side effects and normal post-treatment responses
- Limitations of results
A signed informed consent form confirms that the patient understands the procedure and agrees to treatment within its aesthetic scope.
Managing patient expectations
Practitioners should clearly communicate that:
- Results may be progressive rather than immediate
- Multiple sessions or maintenance treatments may be required
- Individual outcomes vary based on age, skin condition, and lifestyle
Managing expectations reduces dissatisfaction and supports long-term patient trust.
When to postpone or refuse treatment
Treatment should be postponed or declined if:
- Contraindications are present
- The treatment area shows active inflammation or injury
- The patient’s expectations are unrealistic or inconsistent with aesthetic outcomes
Professional judgment should always take precedence over commercial considerations.
7. Clinical Use Guidelines for Safe Operation
Volnewmer RF is designed for professional clinical environments. Safe operation depends on correct training, proper treatment conditions, and adherence to recommended protocols.
7.1 Operator Training
Professional use only
Volnewmer RF is intended exclusively for use by trained professionals in aesthetic clinics or medical spa environments. It is not designed for home or consumer use.
Proper understanding of RF energy behavior
Operators should understand:
- How RF energy interacts with tissue resistance
- The relationship between energy settings and thermal response
- The importance of continuous movement and uniform coverage
This knowledge is essential to avoid uneven heating and ensure consistent results.
Gradual energy escalation protocols
Energy levels should be increased gradualmente, based on:
- Patient tolerance
- Skin response
- Treatment area sensitivity
Avoiding sudden high-energy settings helps reduce discomfort and surface overheating.
7.2 Treatment Environment
Use of conductive gel
A sufficient amount of conductive gel must be applied to:
- Ensure uniform energy transmission
- Reduce surface resistance
- Improve patient comfort
Dry treatment without gel should be avoided.
Proper grounding and electrode placement
Correct placement of grounding components and electrodes is essential to:
- Complete the monopolar RF circuit
- Maintain stable energy flow
- Prevent localized energy concentration
Operators should follow manufacturer instructions for setup and positioning.
Continuous patient feedback during treatment
Patients should be encouraged to communicate sensations during treatment. Practitioners should monitor:
- Heat perception
- Discomfort levels
- Skin response
Immediate adjustments should be made if excessive heat or discomfort is reported.
7.3 Post-Treatment Care
No downtime in most cases
Most patients can resume normal daily activities immediately after treatment.
Temporary erythema or warmth as a normal response
Mild redness, warmth, or slight swelling may occur and typically resolves within hours.
Basic post-treatment skin care advice
Patients are generally advised to:
- Keep the skin hydrated
- Avoid excessive heat exposure on the day of treatment
- Use gentle skincare products for 24 hours
8. Common Side Effects and Expected Reactions
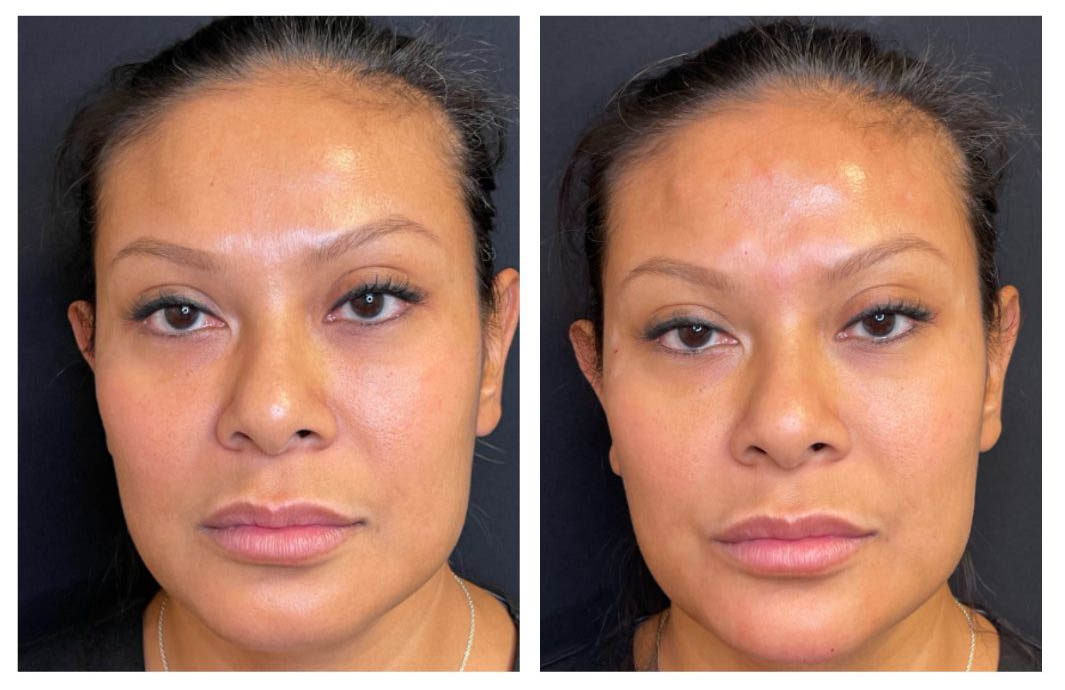

When Volnewmer RF is used according to manufacturer guidelines and proper clinical protocols, side effects are generally mild, predictable, and temporary. These responses reflect normal physiological reactions to controlled dermal heating rather than complications.
Mild redness (erythema)
Temporary redness in the treated area is common and indicates increased blood circulation due to thermal stimulation. This reaction is typically uniform and superficial.
Temporary warmth
Patients may experience a sensation of warmth in the treated area immediately after the procedure. This is a normal response to RF-induced tissue heating and usually subsides shortly after treatment.
Slight swelling in sensitive areas
In areas with thinner skin or higher vascularity—such as the periorbital region or jawline—mild swelling may occur. This swelling is usually minimal and self-limiting.
Typically resolves within hours
In most cases, these effects resolve within a few hours without intervention. Extended downtime is not expected, and patients can generally return to normal daily activities immediately.
Clinics should advise patients that these reactions are normal and temporary, while also instructing them to report any unexpected or prolonged symptoms.
9. RF Safety Compared to Other Aesthetic Technologies

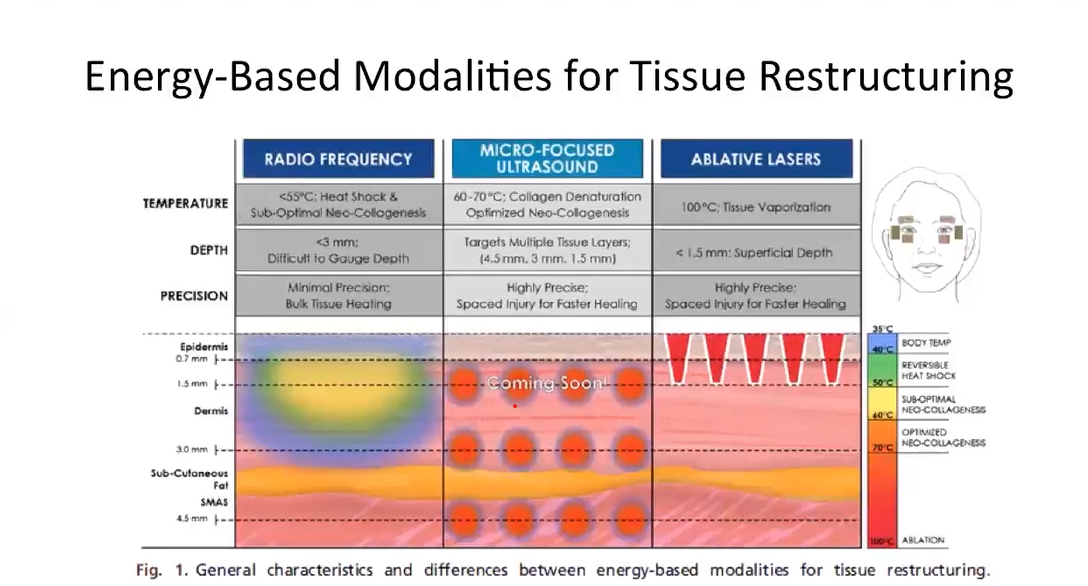

Understanding how radiofrequency compares to other aesthetic technologies helps clinics choose the most appropriate and safest modality for each patient. Volnewmer RF offers distinct safety characteristics when compared to laser, HIFU, and microneedling treatments.
RF vs Laser (Melanin-Independent Technology)
Laser devices rely on light absorption by chromophores, such as melanin or hemoglobin. This can increase the risk of pigment-related complications, particularly in darker skin types.
In contrast, RF technology:
- È melanin-independent
- Does not target skin pigment
- Can be used safely across all Fitzpatrick skin types when protocols are followed
This makes RF an inclusive option for clinics treating diverse patient populations.
RF vs HIFU (No Focal Coagulation Points)
HIFU delivers energy to precise focal points, creating thermal coagulation zones at specific depths. While effective for lifting, this focused energy can be associated with:
- Higher discomfort levels
- Transient nerve sensitivity in certain areas
Monopolar RF:
- Consegna uniform volumetric heating
- Does not create focal coagulation points
- Offers a smoother thermal profile with high patient tolerance
As a result, RF is often preferred for skin quality improvement and maintenance treatments.
RF vs Microneedling (No Skin Barrier Disruption)
Microneedling involves controlled skin penetration to stimulate collagen production. While effective, it:
- Disrupts the skin barrier
- Carries a higher risk of infection if not managed properly
- Requires post-procedure wound care
Volnewmer RF:
- Does not penetrate the skin
- Maintains the integrity of the epidermal barrier
- Reduces infection and cross-contamination risk
- Requires minimal aftercare
10. Why Clinics Trust Volnewmer RF
Clinics choose Volnewmer RF not only for treatment effectiveness, but for the predictability, transparency, and manufacturer accountability behind the system. As the factory and brand owner, Mico Aes has designed Volnewmer RF to meet the real-world needs of professional aesthetic practices.
Established RF technology with predictable outcomes
Volnewmer is based on well-understood monopolar RF principles that clinics already trust. This familiarity reduces the learning curve for practitioners and supports consistent clinical results.
Clear regulatory positioning
Volnewmer RF is clearly positioned as a non-medical aesthetic device, supported by CE documentation and ISO-compliant manufacturing processes. This clarity helps clinics:
- Communicate accurately with patients
- Avoid regulatory ambiguity
- Maintain compliance during inspections or audits
High patient acceptance and comfort
Because treatments are non-invasive, needle-free, and require little to no downtime, patient acceptance is high. This supports:
- Strong word-of-mouth referrals
- Repeat maintenance treatments
- Long-term treatment programs
Suitable for repeated and maintenance use
Volnewmer RF can be integrated into long-term skin management plans, making it ideal for:
- Initial skin tightening programs
- Periodic maintenance treatments
- Combination protocols with HIFU, lasers, or injectables
Direct manufacturer support from Mico Aes
As the factory, Mico Aes provides:
- Complete technical documentation
- Operator training materials
- Safety and contraindication guidelines
- After-sales technical support
This manufacturer-level support gives clinics confidence in both daily operation and long-term device ownership.
11. Conclusion: A Safe, Professional RF Solution When Used Correctly
Volnewmer RF is designed as a professional, clinic-grade monopolar radiofrequency system, with safety built into every stage—from technology selection and manufacturing standards to clinical use guidelines.
Its safety profile is based on:
- Proven monopolar RF physics
- Melanin-independent energy delivery
- Non-invasive, needle-free treatment
- Clear CE non-medical aesthetic classification
- Structured contraindication and screening protocols
When used by trained professionals, following proper patient selection, energy settings, and operating procedures, Volnewmer RF delivers effective skin tightening and rejuvenation with predictable safety and high patient satisfaction.
As the manufacturer, Mico Aes emphasizes that device safety is not defined by technology alone, but by responsible clinical use, proper training, and transparent communication with both practitioners and patients.
Volnewmer RF represents a reliable solution for clinics seeking:
- Advanced monopolar RF performance
- Low-risk, non-invasive treatments
- Sustainable clinical outcomes
- Strong manufacturer backing
For professional aesthetic practices focused on safety, compliance, and long-term growth, Volnewmer RF is a solution designed to support confident, responsible care.











